In 1977, Sir Richard Peto, FRS, FAACR, postulated that larger animals with longer lifespans should develop cancer more readily than their smaller, shorter-lived companions. Since cancer is driven largely by errors in DNA replication, animals with more cells and more time to accumulate mutations should also develop more cancer, he argued.
He observed, however, that this does not appear to be the case. Why, then, do most elephants, whales, and bears get less cancer than humans?
The finding became known as “Peto’s paradox,” a challenge to comparative oncologists to learn more about cancer in vertebrates and understand why cancer incidence doesn’t correlate directly with mass and longevity. Researchers speculated that perhaps understanding the evolutionary processes that prevent cancer in other animals could help us learn how to prevent or treat it in humans.
Work on that front has produced some intriguing findings. In 2015, for instance, researchers found that elephants have 19 extra copies of the tumor suppressor gene TP53 to help them ward off cancer.
But there’s more to cancer risk than genetics, explained Zachary Compton, PhD, a postdoctoral fellow in the University of Arizona Cancer Center’s NCI T32 fellowship program and first author of a recent study in Cancer Discovery, a journal of the American Association for Cancer Research (AACR). “Evolution has all of these crazy stochastic ways it can deal with the same problem,” Compton said. “You don’t expect there to be just one way of handling cancer across the diversity of life.”
In the study, Compton and colleagues analyzed cancer incidence in nearly 300 vertebrate species to identify characteristics associated with cancer prevalence in animals. In addition to finding more species with incredibly high or low cancer rates to study further, the researchers demonstrated a smidge of support for Peto’s original hypothesis and hinted at an explanation for the apparent paradox.
The Pressures of Natural Selection
Compton is particularly interested in the evolutionary underpinnings of cancer. The constraints of natural selection suggest that cancer would be selected against during the evolutionary development of a species.
“Somatic dysfunction is going to decrease the fitness of an individual,” Compton said. “If you have to pump energy into cells that are not working correctly … you would expect natural selection to prune that.”

But traits like body mass, longevity, and gestation time are also subject to selection pressure, Compton explained. With all of these traits intertwined, it can be difficult to tease apart the push and pull of different selective advantages and how they may drive or suppress cancer.
Such an endeavor requires a large sample size, for starters. Compton and colleagues collected 16,049 necropsy records from 292 different animal species at 99 animal care institutions in the United States and London. The records were derived from institutions bound by accreditation requirements to perform a necropsy on every animal that dies in their care, whether from known causes or simply old age.
“All of these deceased animals have had a really thorough investigation by a veterinary pathologist to determine … not only what they died from but what they died with,” Compton explained.
The researchers assessed whether neoplasia (uncontrolled cell growth) was present and in what tissue, and they developed a terminology dictionary based on the neoplasia descriptions to predict whether neoplasms were benign or malignant. Across all species, each of which had a minimum of 20 necropsy records, the median neoplasia prevalence was 4.89%, and the median malignancy prevalence was 3.2%.
Further, Compton and colleagues evaluated the associations between evolutionary traits—including maximum lifespan, adult body mass, basal metabolic rate, gestation length, litter size, time to sexual maturity, and growth rate—and the prevalence of neoplasia and malignancy.
Larger body mass was significantly associated with a higher prevalence of neoplasia; for every tenfold increase in body mass, the risk of neoplasia increased by 2.1%. Other factors associated with malignancy and/or neoplasia included increased maximum longevity, larger litter size in mammals, and, in a subset analysis of 15 species, somatic mutation rate, or the rate at which new mutations occur in the body.
Longer gestation time was significantly associated with a lower prevalence of both neoplasia (5.3% decrease in neoplasia risk per tenfold increase in months of gestation) and malignancy (5.65% decrease in malignancy risk per tenfold increase in months of gestation). Compton speculated that for a large animal to develop rapidly in the womb (or an egg), they must possess mechanisms to suspend natural growth restriction programs—mechanisms that could potentially be exploited later to form cancer.
Because both longevity and gestation time are generally associated with body mass, the researchers performed additional analyses in which they normalized data based on these factors. Body mass was more strongly associated with neoplasia and malignancy prevalence when accounting for gestation time, and vice versa.
Compton and colleagues also addressed the notion that animals in captivity may live longer than nature intended and thus be more susceptible to cancer. In this study, the vast majority of species typically developed tumors within their natural lifespan, and age at death only correlated with higher rates of malignancy in amphibians.
“We not only controlled for natural lifespan, but we discovered that we didn’t need to control for it,” Compton said. “The rates [of neoplasia] we were seeing weren’t because animals live longer in zoos.”
Species ‘Leaderboards’ for Future Research
A component of the study that Compton found particularly exciting was the identification of animals with unusually high or low levels of neoplasia prevalence—a “leaderboard” of species for other researchers to explore. He hopes that animals particularly resistant to neoplasia or malignancy can provide clues to cancer prevention mechanisms that can be repurposed in humans. Similarly, species with abundant cancer may point to genetic, viral, or environmental causes that we can model to study analogous cancer drivers in humans.
Overall, the median prevalence of neoplasia and malignancy varied among taxonomic orders, from 12% and 7%, respectively, in mammals to 1.2% and 0%, respectively, in amphibians. Still, there were outliers in each group.
“It’s not like all of the large mammals have low cancer … or a certain group of amphibians or reptiles,” Compton said. “There are representative species from all of these clades that seem to be very good at preventing cancer.”
Mammals with exceptionally low levels of neoplasia included the Nubian ibex, the tammar wallaby, and several species of bats. Many species of birds appeared particularly resistant to neoplasia, including the black-footed penguin and common songbirds like finches and starlings. Lizards such as the plumed basilisk and the chameleon forest dragon, plus a handful of snakes, also had no neoplasia detected.
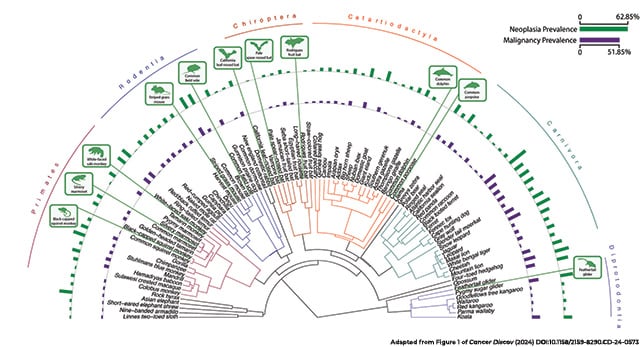
On the other end of the leaderboard, ferrets and opossums had the highest risks of neoplasia—63% and 56%, respectively. Rounding out the top 10 list for neoplasia prevalence were the Dominican mountain chicken frog, jaguar, four-toed hedgehog, Asian elephant, pygmy sugar glider, milk snake, common mouse, and chimpanzee.
Where Do Humans Stack Up?
According to federal statistics, approximately 39.3% of Americans will be diagnosed with cancer during their lifetime, but Compton warned that we may not know the exact prevalence of malignancy in humans. Most natural human deaths do not warrant autopsies, and there is currently no robust way to estimate benign neoplasia at the population level.
But Compton hopes that the data from this study may help researchers better understand and model cancer development and cancer suppression mechanisms that may be translatable to humans. Then, perhaps, we can decrease our own species’ cancer incidence rate or make the disease less deadly.
This study, Compton emphasized, demonstrated that the breakthrough findings about p53 in elephants was merely a starting point for comparative oncologists. “There is so much that nature has to teach us about cancer risk and what controls it,” he said.
The post Why Elephants Don’t Get Cancer but Ferrets Do: Cancer Prevalence Across Vertebrate Animals appeared first on American Association for Cancer Research (AACR).



