Recently approved therapeutics and novel data about optimizing treatment strategies are offering new options for the more than 22,000 patients diagnosed with esophageal cancer each year.
While these cancers are extremely rare, almost 80% of patients die of their disease within five years of diagnosis—underscoring the need for more effective approaches to clinical management. Fortunately, cancer research advances are helping the treatment landscape evolve, with a new first-line treatment approved for esophageal cancer last month and novel insights about when—and even if—to use certain treatments.
Enabling the Patient’s Immune System to Attack Their Esophageal Cancer
Although our immune systems are naturally equipped with the ability to find and kill cancer cells, cancers have ingenious ways to evade detection, including exploiting a system that helps normal cells regulate immune function. Many cancer cells can hijack this safeguard and use it for their own benefit—keeping immune cells “off” so they can’t attack cancer cells.
A class of immunotherapy known as immune checkpoint inhibitors help switch immune cells back “on” and are available for many cancer types. Four immune checkpoint inhibitors—pembrolizumab (Keytruda), nivolumab (Opdivo), ipilimumab (Yervoy), and tislelizumab (Tevimbra)—have been approved by the U.S. Food and Drug Administration (FDA) to treat esophageal cancers in recent years. Initially greenlit for patients with advanced cases that progressed after other treatments, ongoing progress is enabling immune checkpoint inhibitors to treat newly diagnosed patients and those with early-stage disease.
As one recent example of this headway, tislelizumab received its first FDA approval last year for patients with advanced esophageal cancers that progressed during or after prior chemotherapy. And last month, its approval was expanded, allowing it to also be used alongside chemotherapy in newly diagnosed patients who have not yet undergone any treatment. This new first-line approval makes tislelizumab available to even more patients with esophageal cancer.
Using Immunotherapy to Make More Esophageal Tumors Eligible for Surgery
As immunotherapy’s role in the treatment of esophageal cancer continues to grow, understanding when in the treatment course to use it can uncover new opportunities to improve outcomes. A study published in the AACR journal Clinical Cancer Research in November 2024 showed that adding tislelizumab to standard-of-care chemotherapy and radiation shrank unresectable esophageal tumors enough to make them eligible for surgical resection, which, in turn, led to longer patient survival.
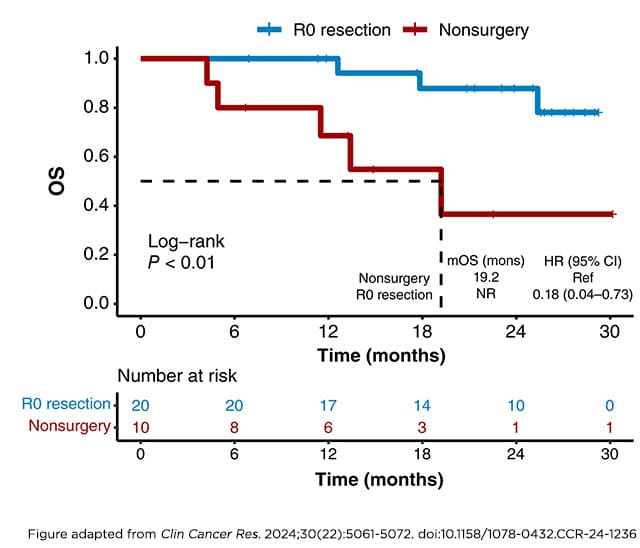
In the phase II clinical trial, 24 patients with unresectable esophageal cancer received presurgical chemotherapy plus radiation, followed by chemotherapy plus tislelizumab. Twenty of these patients had their tumors shrink enough to become eligible for surgery, and 13 had their tumors disappear entirely. Compared with patients who could not undergo surgery, those who underwent surgery had an 82% lower risk of death and a 72% lower risk of disease progression during the year after surgery.
“The [presurgical] treatment approach we tested has the potential to make initially unresectable tumors resectable, giving patients the opportunity to have a durable cancer-free state,” the study’s authors noted in an AACR press release.
Avoiding Unnecessary Surgery for Esophageal Cancer
Conversely, new data suggest that some patients with esophageal cancer may not have added benefit from surgery, which means they could forego the procedure without negatively impacting their outcomes. Results from a phase III clinical trial published this month in Lancet Oncology showed that patients whose locally advanced esophageal tumors were undetectable after chemotherapy and radiation had similar survival rates whether or not they underwent subsequent surgery.
Two years after surgery, 74% of patients who skipped surgery and 71% of those who had the surgery were alive, a difference that was not statistically significant. While extended follow-up is needed to evaluate the long-term impacts of foregoing surgery, the published results may inform treatment decisions for some patients with esophageal cancer.
Esophageal cancer is one of the many rare cancers that, collectively, account for more than 25% of all cancers in the United States. Due to their rarity, these diseases are particularly difficult to study. The upcoming AACR Annual Meeting 2025, April 25-30 in Chicago, will feature sessions that discuss strategies to overcome some of these challenges and empower breakthroughs. Visit the Online Itinerary Planner to learn more.
The post New Treatment Strategies for Esophageal Cancer appeared first on American Association for Cancer Research (AACR).