As the deadliest cancer in the United States, lung cancer is expected to account for approximately 125,070 deaths in 2024 alone. Fortunately, researchers continue to make strides in developing and testing therapeutics, and these efforts have led to 11 new approvals by the U.S. Food and Drug Administration (FDA) for lung cancer so far this year.
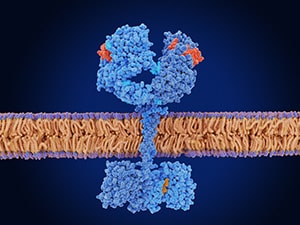
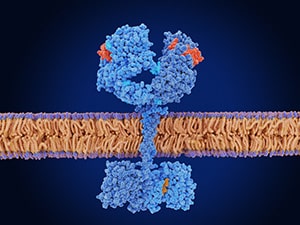
Among the new approvals were several for therapies targeting the epidermal growth factor receptor (EGFR), a cell surface protein that promotes cell division and is mutated in about 20% to 50% of non-small cell lung cancers (NSCLC), the most common type of lung cancer. EGFR mutations, including the prevalent exon 19 deletion and exon 21 L858R substitution, put the protein into overdrive and cause dysregulated cell division and cancer development.
The first-generation EGFR inhibitors were approved in the early 2000s for patients with late-stage NSCLC. However, most patients’ tumors eventually became resistant to these inhibitors, so second- and third-generation EGFR inhibitors were developed to overcome some of the common mechanisms of resistance, as summarized in a previous blog post.
In recent years, researchers and regulatory agencies have expanded the reach of EGFR inhibitors, making them available to additional patients including those with early-stage or newly diagnosed lung cancers.
Osimertinib for Early-stage NSCLC
Osimertinib (Tagrisso) is a third-generation EGFR inhibitor that targets common EGFR mutants as well as T790M, an acquired EGFR mutation that confers resistance to earlier-generation EGFR inhibitors. Originally approved for metastatic NSCLC, the therapeutic has been available to patients with nonmetastatic EGFR-mutated NSCLC since 2020—first as adjuvant therapy for patients with resectable stage 1, 2, or 3 tumors and more recently for patients with stage 3 tumors that cannot be surgically removed.
The expanded indications for osimertinib have benefited many patients with early-stage disease, including Daniel West, who received adjuvant osimertinib to treat his stage 2B NSCLC. West, whose cancer is now in remission, shared his story in the American Association for Cancer Research (AACR) Cancer Disparities Progress Report 2024.
Inhibition of a Less Common EGFR Mutant in Lung Cancers
Most FDA-approved EGFR inhibitors target EGFR with the exon 19 deletion or exon 21 L858R mutations, but approximately 9% of EGFR mutations occur in exon 20. For many years, patients whose NSCLC harbored this less common EGFR mutation were not eligible for EGFR inhibitor therapy. That changed in 2021, when two EGFR inhibitors, amivantamab (Rybrevant) and mobocertinib (Exkivity), received approval to treat locally advanced or metastatic tumors with EGFR exon 20 mutations after progression on chemotherapy.
Unlike most approved EGFR inhibitors, which are small molecule inhibitors or monoclonal antibodies, amivantamab is a bispecific antibody that works by simultaneously binding and blocking the activity of EGFR and the MET protein. Since MET activation is one way cancer cells circumvent EGFR inhibition, the combined block is meant to delay or prevent treatment resistance.
New Combinations for Treatment of Advanced NSCLC
Researchers have also made advances in delaying treatment resistance or improving responses by combining EGFR inhibitors with other therapeutics. This has led to the approval of several new combinations for the treatment of locally advanced or metastatic NSCLC, including in the first-line setting.
This includes a regimen combining the EGFR inhibitor erlotinib (Tarceva) with the angiogenesis inhibitor ramucirumab (Cyramza) for newly diagnosed NSCLCs harboring exon 19 deletion or exon 21 L858R EGFR mutations. Ramucirumab inhibits the vascular endothelial growth factor receptor 2 (VEGFR2), whose activity has been shown to drive resistance to EGFR inhibition; by inhibiting both proteins, the combination suppresses this resistance mechanism.
Combinations involving amivantamab have also been approved to treat locally advanced or metastatic NSCLC. One approved regimen combines amivantamab with another EGFR inhibitor, lazertinib (Lazcluze), for newly diagnosed tumors with the exon 19 deletion or exon 21 L858R mutations. The combined inhibition of EGFR (intracellularly by lazertinib and extracellularly by amivantamab) and MET (by amivantamab) is intended to overcome MET-dependent treatment resistance. Two other recent approvals greenlit amivantamab in combination with chemotherapy for either newly diagnosed NSCLC harboring exon 20 insertion mutations or NSCLC with exon 19 deletion or exon 21 L858R mutations that progressed after prior EGFR inhibition.
In addition, the FDA approved osimertinib in combination with platinum-based chemotherapy as first-line therapy for certain locally advanced or metastatic tumors. The approval was based on clinical trial results that showed greater outcomes with the combination than with osimertinib alone.
Together, these advances illustrate how cancer research is driving progress and making effective treatments available to even more patients. As researchers continue to uncover new insights into EGFR-driven cancers and their resistance mechanisms, we will hopefully see improved outcomes and longer survival for all patients with EGFR-mutated lung cancer.
The post EGFR Inhibitors Extend Their Reach in Lung Cancer appeared first on American Association for Cancer Research (AACR).