As we close out the month of love, the Cancer Research Catalyst staff (chiefly based in the City of Brotherly Love) is not so secretly admiring this month’s Editors’ Picks. February’s selections from the editors of the 10 American Association for Cancer Research (AACR) journals have even formed couples, giving us two studies on improving CAR T-cell efficacy and specificity, two studies on breast cancer risk, and two studies about manipulating the tumor microenvironment.
As always, these studies will be freely available for a limited time.
Journal: Blood Cancer Discovery
Chimeric antigen receptor (CAR) T-cell therapy has remarkably succeeded in treating lymphoblastic leukemia. However, its success in acute myeloid leukemia (AML) remains elusive because of the risk of on-target off-tumor toxicity to hematopoietic stem/progenitor cells (HSPC) and insufficient T-cell persistence and longevity. Using a SynNotch circuit, we generated a high-precision “IF-THEN” gated logical circuit against the combination of CD33 and CD123 AML antigens and demonstrated antitumor efficacy against AML cell lines and patient-derived xenografts. Unlike constitutively expressed CD123 CAR-T cells, those expressed through the CD33 SynNotch circuit could preserve HSPCs and lower the risk of on-target off-tumor hematopoietic toxicity. These gated CAR-T cells exhibited lower expression of exhaustion markers (PD-1, TIM-3, LAG-3, and CD39), higher frequency of memory T cells (CD62L+CD45RA+), and enhanced expansion. Although targeting AML, the moderated circuit CAR signal also helped mitigate cytokine release syndrome, potentially addressing one of the ongoing challenges in CAR-T immunotherapy.
Significance: Our study demonstrates the use of “IF-THEN” SynNotch-gated CAR-T cells targeting CD33 and CD123 in AML reduces off-tumor toxicity. This strategy enhances T-cell phenotype, improves expansion, preserves HSPCs, and mitigates cytokine release syndrome—addressing critical limitations of existing AML CAR-T therapies.
This study was highlighted and featured on the cover of the January issue.
Journal: Cancer Discovery
The Evolutionary Forest of Pancreatic Cancer

The genomic features of pancreatic ductal adenocarcinoma (PDAC) have been well described, yet the evolutionary contexts within which these features occur remain unexplored. We studied genome landscapes, phylogenies, and clonal compositions of 91 PDACs in relation to clinicopathologic features. There was no difference in the number of driver mutations or evolutionary timing when each mutation occurred. High truncal density, a metric of the accumulation of somatic mutations in the lineage that gave rise to each PDAC, was significantly associated with worse overall survival. Polyclonal, monoclonal, or mixed polyclonal/monoclonal metastases were identified across the cohort, highlighting multiple forms of intertumoral heterogeneity. Advanced stage and treated PDACs had higher odds of being polyclonal, whereas oligometastatic PDACs had fewer driver alterations, a lower fractional allelic loss, and increased likelihood of being monoclonal. In sum, our findings reveal novel insights into the dynamic nature of the PDAC genome beyond established genetic paradigms.
Significance: Although the pancreatic cancer genome has been described, it has not been explored with respect to stages of diagnosis or treatment bottlenecks. We now describe and quantify the genomic features of PDAC in the context of evolutionary metrics and in doing so have identified a novel prognostic biomarker.
This article was highlighted and featured on the cover of the February issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Evaluation of Multiple Breast Cancer Polygenic Risk Score Panels in Women of Latin American Heritage
Background: A substantial portion of the genetic predisposition for breast cancer is explained by multiple common genetic variants of relatively small effect. A subset of these variants, which have been identified mostly in individuals of European (EUR) and Asian ancestries, have been combined to construct a polygenic risk score (PRS) to predict breast cancer risk, but the prediction accuracy of existing PRSs in Hispanic/Latinx individuals (H/L) remain relatively low. We assessed the performance of several existing PRS panels with and without addition of H/L-specific variants among self-reported H/L women.
Methods: PRS performance was evaluated using multivariable logistic regression and the area under the ROC curve.
Results: Both EUR and Asian PRSs performed worse in H/L samples compared with original reports. The best EUR PRS performed better than the best Asian PRS in pooled H/L samples. EUR PRSs had decreased performance with increasing Indigenous American (IA) ancestry, while Asian PRSs had increased performance with increasing IA ancestry. The addition of two H/L SNPs increased performance for all PRSs, most notably in the samples with high IA ancestry, and did not impact the performance of PRSs in individuals with lower IA ancestry.
Conclusions: A single PRS that incorporates risk variants relevant to the multiple ancestral components of individuals from Latin America, instead of a set of ancestry-specific panels, could be used in clinical practice.
Impact: The results highlight the importance of population-specific discovery and suggest a straightforward approach to integrate ancestry-specific variants into PRSs for clinical application.
This article was highlighted in the February issue.
Journal: Cancer Immunology Research
Novel therapeutic strategies are needed to improve the efficacy of chimeric antigen receptor (CAR) T cells as a treatment of solid tumors. Multiple tumor microenvironmental factors are thought to contribute to resistance to CAR T-cell therapy in solid tumors, and appropriate model systems to identify and examine these factors using clinically relevant biospecimens are limited. In this study, we examined the activity of B7-H3–directed CAR T cells (B7-H3.CAR-T) using 3D microfluidic cultures of patient-derived organotypic tumor spheroids (PDOTS) and then confirmed the activity of B7-H3.CAR T cells in PDOTS. Although B7-H3 expression in PDOTS was associated with B7-H3.CAR-T sensitivity, mechanistic studies revealed dynamic upregulation of co-inhibitory receptors on CAR T-cells following target cell encounter that led to CAR T-cell dysfunction and limited efficacy against B7-H3–expressing tumors. PD-1 blockade restored CAR T-cell activity in monotypic and organotypic tumor spheroids with improved tumor control and upregulation of effector cytokines. Given the emerging role of TANK-binding kinase 1 (TBK1) as an immune evasion gene, we examined the effect of TBK1 inhibition on CAR T-cell efficacy. Similar to PD-1 blockade, TBK1 inhibition restored CAR T-cell activity in monotypic and organotypic tumor spheroids, prevented CAR T-cell dysfunction, and enhanced CAR T-cell proliferation. Inhibition or deletion of TBK1 also enhanced the sensitivity of cancer cells to immune-mediated killing. Taken together, our results demonstrate the feasibility and utility of ex vivo profiling of CAR T cells using PDOTS and suggest that targeting TBK1 could be used to enhance CAR T-cell efficacy by overcoming tumor-intrinsic and -extrinsic resistance mechanisms.
Journal: Cancer Prevention Research
The German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC) has successfully implemented risk-adapted breast cancer surveillance for women at high breast cancer risk in Germany. Women with a family history of breast and ovarian cancer but without pathogenic germline variants in recognized breast cancer risk genes are recommended annual breast imaging if their predicted 10-year breast cancer risk is 5% or higher, using the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) breast cancer risk model, as outlined in the current GC-HBOC guideline. However, women who initially do not meet this risk threshold may do so later, even if there is no new cancer in their family. To determine when this threshold is crossed, one could annually repeat BOADICEA calculations using an aging pedigree: the “prediction by aging pedigree” (AP) approach. Alternatively, we propose a simplified and more practical “’conditional probability” (CP) approach, which calculates future risks based on the initial BOADICEA assessment. Using data from 6,661 women registered with GC-HBOC, both methods were compared. Initially, 74% of women, ages 30 to 48 years, had a 10-year breast cancer risk below 5%, but 53% exceeded this threshold at an older age based on the AP approach. Among the women with an initial risk below the threshold, the CP approach revealed that 99% of women exceeded the 5% threshold at the same or an earlier age compared with the AP approach (88% of cases were within the same year or 1 year earlier). The CP approach has been implemented as a user-friendly web application.
Prevention Relevance: The German Consortium for Hereditary Breast Cancer recommends annual breast imaging for women if their 10-year breast cancer risk is 5% or higher. Women who initially do not meet this risk threshold may do so later. We propose a simple method to determine future risks based on initial risk assessments.
Journal: Cancer Research (February 1 issue)
Precision medicine approaches to cancer treatment aim to exploit genomic alterations that are specific to individual patients to tailor therapeutic strategies. Yet, some targetable genes and pathways are essential for tumor cell viability even in the absence of direct genomic alterations. In underrepresented populations, the mutational landscape and determinants of response to existing therapies are poorly characterized because of limited inclusion in clinical trials and studies. One way to reveal tumor essential genes is with genetic screens. Most screens are conducted on cell lines that bear little resemblance to patient tumors, after years of culture under nonphysiologic conditions. To address this problem, we aimed to develop a CRISPR screening pipeline in three-dimensionally grown patient-derived tumor organoid (PDTO) models. A breast cancer PDTO biobank that focused on underrepresented populations, including West African patients, was established and used to conduct a negative-selection kinome-focused CRISPR screen to identify kinases essential for organoid growth and potential targets for combination therapy with EGFR or MEK inhibitors. The screen identified several previously unidentified kinase targets, and the combination of FGFR1 and EGFR inhibitors synergized to block organoid proliferation. Together, these data demonstrate the feasibility of CRISPR-based genetic screens in patient-derived tumor models, including PDTOs from underrepresented patients with cancer, and identify targets for cancer therapy.
Significance: Generation of a breast cancer patient-derived tumor organoid biobank focused on underrepresented populations enabled kinome-focused CRISPR screening that identified essential kinases and potential targets for combination therapy with EGFR or MEK inhibitors.
A commentary related to this study was published in the February 1 issue.
Journal: Cancer Research (February 15 issue)
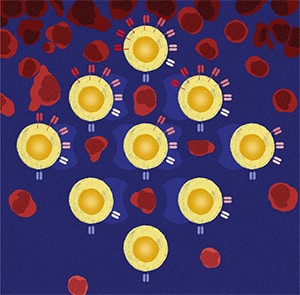
Breast cancer subtypes display different metastatic organotropism. Identification of the mechanisms underlying subtype-specific organotropism could help uncover potential approaches to prevent and treat metastasis. In this study, we found that forkhead box F2 (FOXF2) promoted the seeding and proliferative recovery from dormancy of luminal breast cancer (LumBC) and basal-like breast cancer (BLBC) cells in the bone by activating the NF-κB and BMP signaling pathways. FOXF2 promoted LumBC cell seeding but not proliferative recovery in the lung by activating the BMP signaling pathway. Conversely, FOXF2 suppressed the seeding and proliferative recovery of BLBC cells in the lung by repressing the TGFβ signaling pathway. FOXF2 directly upregulated RelA/p65 transcription and expression in LumBC and BLBC cells by binding to the RELA proximal promoter region and RelA/p65 bound to the FOXF2 proximal promoter region to upregulate expression, forming a positive feedback loop. Targeting the NF-κB pathway efficiently prevented the metastasis of FOXF2-overexpressing breast cancer cells to the bone, whereas inhibiting TGFβ signaling blocked the metastasis of BLBC with low FOXF2 expression to the lung. These findings uncover critical mechanisms of breast cancer subtype–specific organotropism and provide insights into precision assessment and treatment strategies.
Significance: FOXF2 regulates signaling pathways in a subtype-specific manner to coordinate the fate of disseminated breast cancer cells in distant organs, suggesting that FOXF2 functions could be harnessed to prevent organ-specific metastasis.
A commentary related to this study was published in the February 15 issue.
Journal: Clinical Cancer Research (February 1 issue)
Purpose: Effective therapy for recurrent head and neck squamous cell carcinoma (HNSCC) that is refractory to chemotherapy and immunotherapy is a considerable need. Aurora kinase A inhibition leads to apoptosis and immunogenic cell death in preclinical models of human papilloma virus (HPV)–driven cancers.
Patients and Methods: Alisertib was administered orally twice daily on days 1–7 and pembrolizumab on day 1 of a 21-day cycle to adults with advanced solid tumors (phase I) or with immunotherapy- and platinum-resistant, HPV-positive HNSCC (phase II).
Results: The recommended phase II alisertib dose was 40 mg, which had only the expected toxicity including cytopenia that led to dose reductions in two phase II patients at cycles 13 and 16. We saw no objective responses, but the combination led to prolonged stable disease (SD) in several patients, including two of 10 phase I patients (8 and 27 months). Eight of the 15 HPV-positive patients had SD, of which four (heavily pretreated) had ≥6 months, with median overall and progression-free survival durations of 16.8 and 1.4 months, respectively. In circulating immune cells and plasma, patients with SD had markedly higher levels of HLA de novo resistance–expressing NK cells than did progressive disease patients who demonstrated a more immunosuppressive and inflammatory profile. Pharmacokinetics did not indicate any significant drug-drug interactions between pembrolizumab and alisertib.
Conclusions: The combination of alisertib and pembrolizumab was well tolerated and led to prolonged SD in some immunotherapy-resistant patients, supporting our hypothesis that Aurora kinase A inhibition can reverse immunotherapy resistance of retinoblastoma protein–deficient HNSCC.
Journal: Clinical Cancer Research (February 15 issue)
Purpose: Novel combinations are required to overcome resistance to immune checkpoint inhibitors in proficient mismatch repair (pMMR) or microsatellite-stable (MSS) metastatic colorectal cancer (mCRC). We aimed to determine whether vorbipiprant, a prostaglandin E2 receptor EP4 subtype antagonist, can convert immune-resistant mCRC into a tumor responsive to anti–PD-1 inhibition.
Patients and Methods: This phase Ib/IIa prospective, open-label, single-arm trial followed a 3 + 3 dose-escalation and dose-optimization design. A total of 28 patients with chemorefractory pMMR/MSS mCRC were given dose-escalated oral vorbipiprant (30, 90, or 180 mg twice daily), along with biweekly intravenous balstilimab (3 mg/kg), an anti–PD-1 antibody. The primary endpoints included safety and the disease control rate (DCR). Secondary endpoints were the overall response rate, duration of response, progression-free survival, and overall survival.
Results: No dose-limiting toxicities were observed. Of the 28 patients, seven (25%) experienced serious adverse events, but only one was attributed to vorbipiprant and one to balstilimab. The trial achieved a DCR of 50% observed across the entire cohort. In the subgroup of patients with liver metastases (n = 12), the DCR was 25%. The overall response rate was 11%, with three patients showing a partial response (median duration of response, 7.4 months). The median progression-free survival was 2.6 months, and the median overall survival was 14.2 months. Translational exploratory analyses suggested that vorbipiprant may boost response to anti–PD-1 in patients with immunogenic tumors.
Conclusions: The combination of vorbipiprant and a PD-1 inhibitor (balstilimab) yielded sufficient activity in refractory pMMR/MSS mCRC, which is worthy of confirmation in future clinical trials in biomarker-enriched populations.
Journal: Molecular Cancer Research
PRMT5 Maintains Tumor Stem Cells to Promote Pediatric High-Grade Glioma Tumorigenesis
Pediatric high-grade gliomas (PHGG) are aggressive, undifferentiated central nervous system tumors with poor outcomes, for which no standard-of-care drug therapy currently exists. Through a knockdown (KD) screen for epigenetic regulators, we identified PRMT5 as essential for PHGG cell growth. We hypothesized that, similar to its effect in normal cells, PRMT5 promotes self-renewal of stem-like PHGG tumor-initiating cells essential for tumor growth. We conducted in vitro analyses, including limiting dilution studies of self-renewal, to determine the phenotypic effects of PRMT5 KD. We performed chromatin immunoprecipitation sequencing (ChIP-Seq) to identify PRMT5-mediated epigenetic changes and performed gene set enrichment analysis to identify pathways that PRMT5 regulates. Using an orthotopic xenograft model of PHGG, we tracked survival and histologic characteristics resulting from PRMT5 KD or administration of a PRMT5 inhibitor ± radiation therapy. In vitro, PRMT5 KD slowed cell-cycle progression, tumor growth and self-renewal, and altered chromatin occupancy at genes associated with differentiation, tumor formation, and growth. In vivo, PRMT5 KD increased survival and reduced tumor aggressiveness; however, pharmacologic inhibition of PRMT5 with or without radiation therapy did not improve survival. PRMT5 KD epigenetically reduced tumor-initiating cells’ self-renewal, leading to increased survival in preclinical models. Pharmacologic inhibition of PRMT5 enzymatic activity may have failed in vivo due to insufficient reduction of PRMT5 activity by chemical inhibition, or this failure may suggest that nonenzymatic activities of PRMT5 are more relevant.
Implications: PRMT5 maintains and promotes the growth of stem-like cells that initiate and drive tumorigenesis in PHGG.
This study was highlighted in the February issue.
Journal: Molecular Cancer Therapeutics
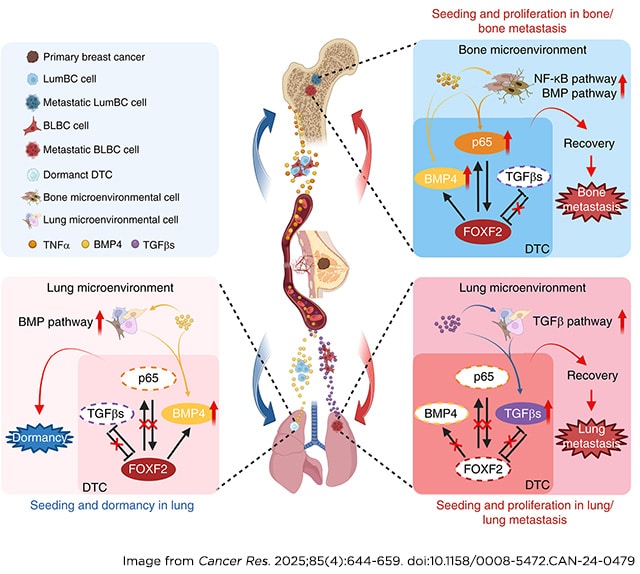
Harnessing the Potential of FAP-IL12mut TMEkine for Targeted and Enhanced Antitumor Responses
Although cancer immunotherapy has yielded encouraging outcomes in hematologic malignancies, it has faced challenges in achieving the same level of effectiveness in numerous solid tumors, primarily because of the presence of immunosuppressive tumor microenvironments (TME). The immunosuppressive qualities of the TME have generated considerable interest, making it a focal point for treatments aimed at enhancing immune responses and inhibiting tumor progression. Fibroblast activation protein (FAP), an attractive candidate for targeted immunotherapy, is prominently expressed in the TME of various solid tumors. IL12, recognized as a key mediator of immune responses, has been explored as a potential candidate for cancer treatment. Nevertheless, initial efforts to administer IL12 systemically demonstrated limited efficacy and notable side effects, emphasizing the necessity for innovation. To address these concerns, our molecules incorporated specific IL12 mutations, called IL12mut, which reduced toxicity. This study explored the therapeutic potential of the FAP-IL12mut TMEkine—a novel immunotherapeutic agent selectively engineered to target FAP-expressing cells in preclinical cancer models. Our preclinical results, conducted across diverse murine cancer models, demonstrated that FAP-IL12mut significantly inhibits tumor growth, enhances immune cell infiltration, and promotes a shift toward a cytotoxic immune activation profile. These findings suggest that FAP-IL12mut could offer effective cancer treatment strategies.
This article was highlighted in the February issue.
Journal: Cancer Research Communications
A Cost-Effective Two-Step Approach for Multi-Cancer Early Detection in High-Risk Populations
In population-wide cancer screening, three key issues need to be focused on: the number of cancer cases identified, the number of false positives, and the cost. OncoSeek is a multi-cancer early detection (MCED) test using seven protein tumor markers and artificial intelligence. SeekInCare is an MCED test that integrates the seven protein tumor markers and four cancer genomic features from cell-free DNA by shallow whole-genome sequencing. In a two-step approach, the initial screening is conducted using OncoSeek, and SeekInCare is then used as the secondary test for individuals who tested positive by OncoSeek. We simulated a screening in five million adults ages ≥50 years with a cancer incidence rate of 1.9%. Whereas at 91.0% specificity OncoSeek had 441,450 false positives, using the two-step approach significantly reduced false positives to 34,335 (0.7%). Although SeekInCare and Galleri identified more cancer cases (32,015 and 27,455, respectively) than the two-step MCED (21,280), their total costs reached $3,750 million and $4,745 million, respectively. As the positive predictive value of two-step MCED (38.3%) was comparable with SeekInCare (27.7%) and Galleri (38.3%), it reduced the cost by 5.3-fold and 6.6-fold, respectively, amounting to a total cost of $713.6 million and a cost of $143 per individual screened. The cost of per cancer case detected was $117,133 for SeekInCare and $172,828 for Galleri, which were 3.5-fold and 5.2-fold higher, respectively, than the two-step MCED ($33,534). The two-step approach not only significantly reduces false positives but also cuts down the screening cost substantially, making it a cost-effective strategy for population-wide cancer screening.
Significance: Large-scale screening inevitably leads to significant financial burdens on the healthcare system, which is a key factor constraining nationwide screenings. The two-step MCED approach not only maintains comparable performance but also substantially alleviates financial strains compared with the direct use of next-generation sequencing–based MCED tests for massive screenings.
The post Editors’ Picks, February 2025: Improving CAR T, Studying Breast Cancer Risk, and More appeared first on American Association for Cancer Research (AACR).